Introduction
When we think of stem cells, many of us imagine secret experiments with embryos or zygotes, often sparking huge ethical debates. I remember that when I was young, there was a stem cell clinic in my town. It seemed mysterious — almost like a confidential organisation. But here’s the twist: actual stem cells are literally everywhere in our lives, making up most of the cells in our bodies. They’re not as mysterious, secretive, or even gory as they might sound.
In this episode, I’ll walk you through what stem cells are.
Photo Credit:

What are stem cells?
So, what exactly are stem cells? Stem cells are cells that can self-renew. Self-renewal means they can divide to replace lost or damaged cells (like when your body heals a wound). Our hair cells, tongue cells, and even skin cells are all stem cells.
Now, at this point, you might think: “Okay… then isn’t almost every cell in our body a stem cell?” Well, not quite! Stem cells have another fascinating property: they can differentiate.
Differentiation means cells can turn into other types of cells. Think of it like university: a student might enter a Bachelor of Science degree without a set major, but by the time they graduate, they’ve chosen genetics, biochemistry, microbiology, molecular biology, or something else. Their identity is eventually defined — just like cells!
Morphogen gradient
As I mentioned earlier, the process of differentiation is kind of like selecting a major at university and then graduating with it. But then, how do cells actually pick their “major”? In other words, who decides what a cell will eventually become?
That’s where the concept of the morphogen gradient comes in. A morphogen is a protein that determines the fate of nearby cells. The difference in concentration of this protein (=the gradient) gives cells positional information and basically tells them: “You, differentiate into this type of cell!” 🧭
I know it sounds a bit complicated, but let’s take an example. In fruit flies (Drosophila), a protein called bicoid is responsible for forming the head. It essentially tells the developing embryo: “This part will be the head, that part the body, and over here the legs!” Just like a traffic police officer directing cars, the morphogen guides cells to become the right type of cell in the right place.
Does that make a bit more sense now? 🙂
Cell potency
Different stem cells have different abilities to transform. This ability is called cell potency. In our bodies, there are four main types: totipotent, pluripotent, multipotent, and unipotent.
- Totipotent 🌟
Totipotent cells can form an entire body — literally any cell type, including extraembryonic tissues like the placenta. After fertilisation, the zygote divides to form the morula, whose cells can eventually create all organs and tissues.
Photo Credit: Ferty 9 Fertility Centre

- Pluripotent 🌈
Pluripotent cells can’t make a whole body, but they can still become many different cell types. In the blastocyst stage, cells form three layers — endoderm, ectoderm, and mesoderm — each responsible for specific organs. But like a restaurant menu with chicken, beef, and vegetarian sections, you need all three to make the full spread.
Photo credit: inviTRA 'Gastrulation and development of organ systems'

- Multipotent 🎯
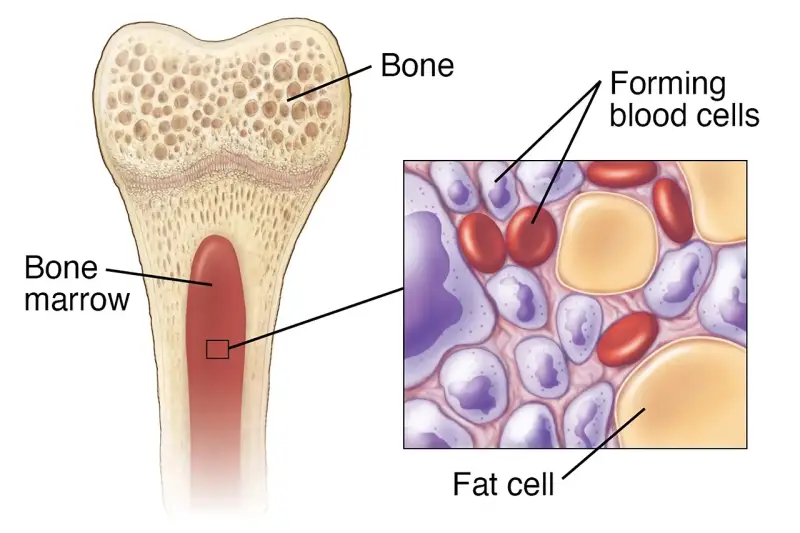
Multipotent cells can transform into closely related types. For instance, bone marrow cells can become different blood cells — red, white, and more. This is why patients with leukaemia often receive bone marrow transplants. (Fun fact: B cells are called “B” because they mature in the Bone marrow!)
Photo credit: WebMD 'Bone Marrow'
- Unipotent 🔒
Unipotent cells can only differentiate into one type of cell, but they can still self-renew. For example, liver cells only make liver cells, but a transplanted liver can regrow in both donor and recipient. Hair cells are also unipotent — they only make hair, but they regenerate endlessly.
Human Embryonic Stem Cells 🫀
Among the many types of stem cells, the ones that receive the most attention are totipotent and pluripotent stem cells. Why? Because their potential in both research and real-world applications is practically limitless! Recently, the concept of organoids—miniature organs grown from stem cells—has gained global attention, along with medical applications for treating degenerative diseases like Parkinson’s and Alzheimer’s.
What’s an organoid?
Organoids are 3D structures cultivated from stem cells that resemble real organs 🫁. They are currently being used in disease modelling, regenerative medicine, drug development, and even personalised medicine.
But… here’s the issue: these stem cells typically only exist during the embryonic stage. In Korea, for example, embryos used for research often come from surplus embryos left over from IVF (in vitro fertilisation). With donor consent, these unused embryos can be utilised in research. However, the number of embryos available is extremely limited due to ethical concerns. On top of that, when applied in therapy, issues like immune rejection still need to be solved. And practically, even isolating the inner cell mass (ICM)—a cluster of cells inside the blastocyst that’s used to derive embryonic stem cells—is very challenging.
To address these ethical problems, research worldwide is now focusing on creating embryo-like structures using pluripotent stem cells, without the need for sperm or eggs. In April 2023, the Chinese Academy of Sciences reported successful results using monkeys, and later in September, the Weizmann Institute in Israel succeeded in creating human embryo–like structures using human pluripotent stem cells.
The idea that embryos can be created without sperm or eggs… strange, right? But also fascinating! 🤯 Although we haven’t yet reached the stage of generating embryo-like structures from induced pluripotent stem cells (iPSCs), it makes you wonder… maybe one day, an embryo with the exact same genetic information as me could be created. (Almost like a real-life Mickey 17 moment!)
Induced Pluripotent Stem Cells (iPS) 🧪
Of course, there have been many efforts to resolve the ethical issues surrounding human embryonic stem cells. But perhaps the best way to overcome those concerns is not to extract these stem cells from embryos at all, but to create them another way.
We can’t literally go back to the embryonic stage — but what if we could “rewind” adult body cells into a pluripotent state? That’s exactly what Induced Pluripotent Stem Cells (iPS) are: adult somatic cells genetically reprogrammed into an “all-purpose mode.” This groundbreaking solution earned Shinya Yamanaka and John Gurdon the 2012 Nobel Prize in Physiology or Medicine.🏆
Photo Credit: News-Medeical.net

How is iPS created?
Now, you might be wondering: How can a mature adult cell, which seems to have nothing to do with embryos, suddenly become a pluripotent stem cell? Long story short: it’s like a time reversal, sending the cell back to an embryonic-like state.
Here’s how it works:
1️⃣ Collect a patient’s cells (commonly skin cells or fibroblasts) → culture them in a dish
2️⃣ Introduce reprogramming factors into the cells
3️⃣ Wait for several weeks
4️⃣ Pluripotent stem cells are generated ✨
5️⃣ Adjust culture conditions to guide them into differentiating into various types of cells
What are these reprogramming factors?
When these special genes are introduced, they “switch off” the fate-setting program of a mature cell, allowing it to reset into a state very similar to embryonic stem cells. It’s almost like a time slip — rewinding the cell’s developmental clock.
Photo Credit: The regeneration centre

Conclusion 💬
Stem cells are everywhere, yet at the same time, they’re truly extraordinary. They are the fundamental building blocks of our bodies and hold the key 🔑 to the future of medicine.
In this article, I focused more on introducing the basic concepts of stem cells rather than going deep into their real-world applications. Still, I hope it gave you a clearer picture of what stem cells are and why they matter. And maybe, just maybe, it sparked a bit of curiosity to learn more about these fascinating cells!
Add comment
Comments